Abstract
Introduction: Since many patients with chronic phase chronic myeloid leukemia (CP CML) achieve long-term survival, health-related quality of life (HR-QOL) as assessed by PROs is an important consideration. Bosutinib is a SRC/ABL tyrosine kinase inhibitor approved for Philadelphia chromosome-positive CML treatment in adults resistant or intolerant to prior therapy. In the BFORE trial (NCT02130557) of bosutinib versus imatinib a significantly higher major molecular response rate at 12 months (the primary endpoint) occurred with bosutinib. Here we report initial PRO results from BFORE.
Methods: BFORE is an ongoing, multinational, phase 3, open-label study in patients with newly diagnosed CP CML. Patients are randomized 1:1 to bosutinib 400 mg once daily or imatinib 400 mg once daily. PROs, assessed as exploratory endpoints, included functional status using the Functional Assessment of Cancer Therapy-Leukemia (FACT-Leu) and the European Quality of Life-5 Dimensions-Visual Analog Scale (EQ-5D-VAS) instruments. Assessments were conducted at baseline and every 3 to 6 months, to continue for up to 5 years after treatment initiation. Outcomes at month 12 are reported herein.
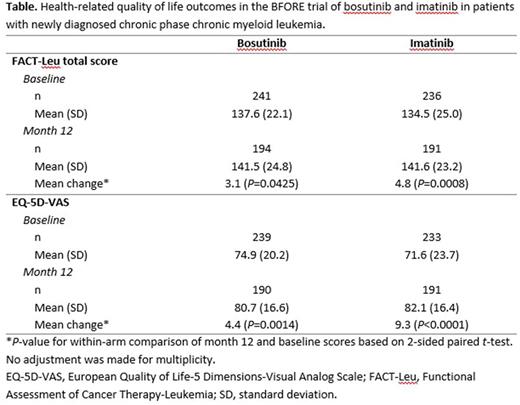
Results: Bosutinib and imatinib mean FACT-Leu scores were similar at baseline and at month 12 (Table). At month 12, significant improvement from baseline in FACT-Leu total score was observed for both bosutinib (mean change 3.1, P=0.0425) and imatinib (mean change 4.8, P=0.0008). For bosutinib at month 12, all FACT-Leu combined and subscale scores demonstrated either improvement (emotional well-being; leukemia symptoms; FACT-Leu total score) or maintenance (physical, functional, social well-being; FACT-General total score; trial outcome index).
Bosutinib and imatinib mean EQ-5D-VAS scores were similar at baseline and at month 12 (Table). At month 12, significant improvement in mean EQ-5D-VAS was observed for both bosutinib (mean change 4.4, P=0.0014) and imatinib (mean change 9.3; P <0.0001). Additional FACT-Leu and EQ-5D analyses yielded similar findings. For example, mean [SD] Functional Health Status as measured using the EQ-5D Utility Score was similar between groups at baseline (bosutinib: 0.688 [0.2710] and imatinib: 0.657 [0.2929]) and improved from baseline for subjects in both treatment arms, with comparable scores seen at Month 12 (bosutinib: 0.722 [0.2953] and imatinib: 0.724 [0.2717]).
Conclusion: In the BFORE trial of first-line CP CML therapy, PROs for bosutinib indicated improvement or maintenance of HRQoL at month 12, with effects comparable to those observed for imatinib, the standard of care for first-line CML treatment. These exploratory PRO results indicate fewer symptoms, better HRQoL, and improved functional health status at month 12 relative to baseline for both therapies.
Cortes: ARIAD: Consultancy, Research Funding; Sun Pharma: Research Funding; Novartis Pharmaceuticals Corporation: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; ImmunoGen: Consultancy, Research Funding; Teva: Research Funding. Gambacorti-Passerini: Pfizer: Consultancy, Honoraria, Research Funding; BMS: Consultancy. Deininger: Novartis: Consultancy, Research Funding; Incyte: Consultancy; ARIAD: Consultancy; Ariad Pharmaceuticals, Bristol Myers Squibb, CTI BioPharma Corp, Gilead, Incyte, Novartis, Pfizer, Celgene, Blue Print, Galena: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy; BMS: Consultancy, Research Funding; Celgene: Research Funding; Gilead: Research Funding. Mauro: Bristol-Myers Squibb: Consultancy. Chuah: Chiltern: Honoraria; BMS: Honoraria, Other: Travel; Novartis: Honoraria; Avillion: Honoraria. Kim: Il-Yang: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding. Milojkovic: ARIAD: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Incyte: Honoraria, Speakers Bureau; BMS: Consultancy, Honoraria. le Coutre: Incyte: Honoraria; BMS: Honoraria; Pfizer: Honoraria; Novartis: Honoraria, Research Funding; ARIAD: Honoraria. García Gutiérrez: BMS: Consultancy, Honoraria, Research Funding; Incyte: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding. Reilly: Avillion LLP: Employment. Jeynes-Ellis: Avillion LLP: Employment, Equity Ownership. Crescenzo: Pfizer: Employment, Equity Ownership. Mamolo: Pfizer: Employment, Equity Ownership. Reisman: Pfizer: Employment. Bardy-Bouxin: Pfizer: Employment, Equity Ownership. Hochhaus: Novartis: Research Funding; Pfizer: Research Funding; Incyte: Research Funding; Ariad: Research Funding; MSD: Research Funding; BMS: Research Funding. Brümmendorf: Pfizer: Consultancy, Research Funding; Novartis: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal